The AABB Zika Virus (ZIKV) Biovigilance Network platform was initiated in 2016 as a collaboration between AABB & U.S. blood collection establishments in response to the FDA’s first guidance on ZIKV, released in February 2016. It was modeled after AABB’s West Nile Virus (WNV) Biovigilance Network, initiated in 2006 by AABB’s WNV Task Force.
The ZIKV Biovigilance Network platform contained collection and reporting of data for donations with reactive ZIKV Nucleic Acid Test (NAT) results and mapped these data to U.S. geographical locations. Enhanced ZIKV reporting platform was deployed in December 2018 to support NAT for ZIKV described in the recommendations (including triggering ID NAT) issued in the July 2018 Food and Drug Administration (FDA) Guidance. AABB presented data from the platform during the March 2019 Blood Products Advisory Committee Meeting. In May 2021, FDA determined that ZIKV is no longer a relevant transfusion-transmitted infection (RTTI) under its regulations.
AABB’s ZIKV Biovigilance Network platform was discontinued on November 15, 2022.
A letter from Sharon Carayiannis, AABB’s Vice President, Science and Practice, to the ZIKV Biovigilance Network participants describes the details.
Data was downloaded on 9/22/2022.
For questions, please contact us at hemovigilance@aabb.org.
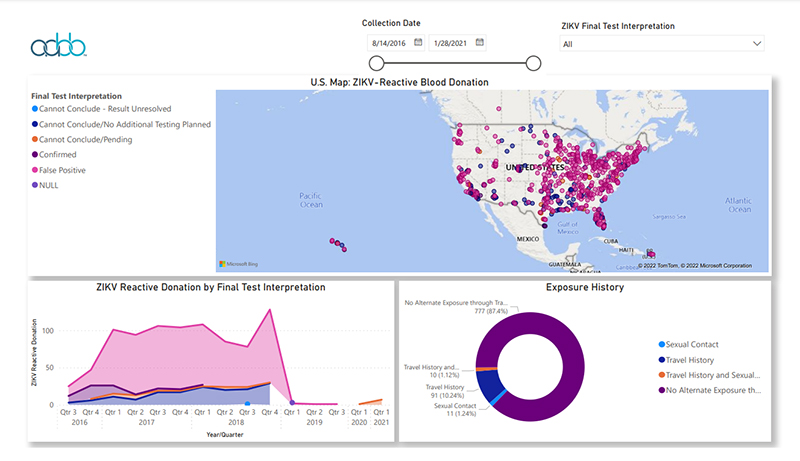 Click
the graph above to view interactive report
Click
the graph above to view interactive report
The data posted on this web site is provided by third parties and is not verified by AABB. Accordingly, AABB makes no guarantee that the data is current, valid or accurate. No content on this site is intended to constitute professional advice, whether medical, legal or otherwise. AABB expressly disclaims any liability or loss that may arise either directly or indirectly from relying on the information, services or other material on this site.
Reporting facilities that are HIPAA-compliant should report only patient data that has been de-identified in accordance with HIPAA de-identification standards. AABB is not responsible for reporting facilities’ compliance with federal, state, or local laws or regulations.