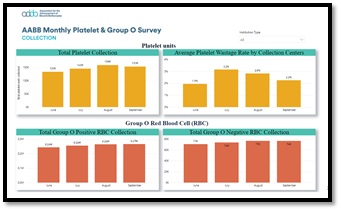
AABB regularly surveys its members for information about blood collection, utilization and current practices. The AABB survey results are shared with AABB membership, who use them for benchmarking and to better understand the current state of the field. Additionally, AABB uses these data to identify industry trends and to inform AABB’s decision making, planning and policy positions.
AABB is building out a robust data infrastructure to better capture important information about the strength and stability of the blood supply and about the practice of transfusion medicine.
This project was born from discussions about the challenges in evaluating the state of the blood supply, particularly with platelets and group O red blood cell components, due to the absence of reliable data. AABB thanks the many participating member facilities for contributing valuable data to the Association’s Monthly Platelet and Group O Survey during the past few years. Your participation helped us collect and publish important data that has been instrumental in improving our community’s understanding of the current blood supply and has been referenced at various influential conferences and committee meetings.
After evaluation of the project data, and with awareness of other developing data resources, AABB has decided to discontinue the “Monthly Collection” section of the survey. Beginning the survey year 2024, we will focus on the blood utilization data. While we understand terminating the collection portion of the survey may affect our understanding of the current blood supply, we assure the community that we will continue to publish the utilization data collected through the project. We will release the survey invitations quarterly requesting participants to provide monthly data.
Below is an interactive report from the survey with basic metrics to identify trends. We hope that, over time, the survey results will evolve and be an important source of data for AABB members and the larger transfusion community. The more data we collect, the better the information we can provide.
Click the graph below to view interactive report: