AABB continues to work with facilities throughout the world on international hemovigilance efforts. Through its expert committees, AABB collaborates with government agencies and international organizations to establish and promote standard definitions related to transfusion reactions. Additionally, AABB committees develop education and support research to further the goals of hemovigilance.
A working group of the AABB Hemovigilance Committee developed four incident codes to assist in the recognition and reporting of instances of under-transfusion due to inventory. The codes, described in a recent report in Transfusion, are:
The proposed incident codes have been incorporated into the Centers for Disease Control and Prevention (CDC), National Healthcare Safety Network (NHSN) Hemovigilance Module. Please refer to the CDC NHSN protocol (page 26).
The sub-group is hopeful that adoption of these codes within the global hemovigilance system will help improve recognition and reporting of instances of under-transfusion due to inventory, thus supporting the development of better collection strategies and inventory management techniques, as well as effective policies to advance blood safety and availability.
Please check the resource section to access the publication.
 The AABB Common Transfusion Reaction Reporting Form is intended for use by hospitals and blood centers to communicate about transfusion reactions, particularly when there are multiple suppliers to the hospital transfusion service. The form helps to streamline the process for hospitals and provide complete information for blood suppliers when investigating transfusion reactions. The AABB Donor Hemovigilance Working Group and Hemovigilance Committee updated version 2.0 of the form to include new risk factors (e.g., COVID-19 related respiratory disease), and update Diagnostics, and Treatment and Clinical Course sections.
The AABB Common Transfusion Reaction Reporting Form is intended for use by hospitals and blood centers to communicate about transfusion reactions, particularly when there are multiple suppliers to the hospital transfusion service. The form helps to streamline the process for hospitals and provide complete information for blood suppliers when investigating transfusion reactions. The AABB Donor Hemovigilance Working Group and Hemovigilance Committee updated version 2.0 of the form to include new risk factors (e.g., COVID-19 related respiratory disease), and update Diagnostics, and Treatment and Clinical Course sections.
Common Transfusion Reaction Reporting Form (Updated)
Common Transfusion Reaction Reporting Form FAQ (Updated)
Provide your Feedback
The revised transfusion associated circulatory overload (TACO) definition (2018) developed and validated by the International Society of Blood Transfusion (ISBT) working party on Haemovigilance in collaboration with the International Haemovigilance Network (IHN) and AABB, is now available online and results of the formal validation have been published (The Lancet Haematology, 2019). The revised definition is applicable to cases that occur up to 12 hours after transfusion. Combinations of signs and symptoms which can add up to meet the surveillance diagnostic criteria will help qualify cases where there may be no chest x-ray and/or record of elevated BNP concentrations as TACO. Notes on signs and symptoms and didactic table listing of key features have been added to assist in making diagnosis. The revision group emphasizes that the chief priority is to adopt standard reporting criteria, which will enable professionals to raise awareness of TACO and lead to improved reporting, research and reduction of transfusion complications.
Transfusion-associated circulatory overload (TACO) Definition (2018)
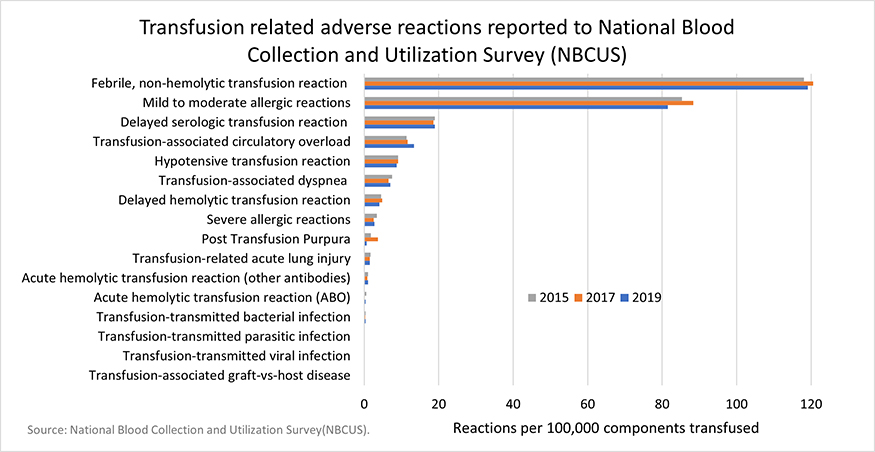
 AABB Quick Reference Guide for NHSN Hemovigilance Module: Adverse Reaction Definitions (Updated)
AABB Quick Reference Guide for NHSN Hemovigilance Module: Adverse Reaction Definitions (Updated)
Save the Date: Next Virtual Journal Club Taking Place May 14-15
April 19, 2024
UK Issues National Patient Safety Alert on Reducing TACO Risk
April 05, 2024
Registration Open for IHN Symposium 2024
March 27, 2024