The Cellular Starting Material (CSM) Qualification is a new recognition for AABB-accredited facilities that want to highlight their expertise, capacity and practices related to collecting CSM (including whole blood for research, byproducts from blood processing, or apheresis mononuclear cells, etc.) to biotech companies and research facilities throughout the world who are looking for qualified suppliers.
This verification is intended to reduce the laborious review and duplicative inspection criteria for both parties and give CSM-qualified facilities a business advantage to establish, strengthen and maintain relationships with biopharma organizations. Ultimately, this recognition program will support the growth and diversification of AABB-accredited facilities.
Biopharma organizations struggle to find and validate qualified CSM supplies needed to ramp-up to commercialization. AABB’s CSM Qualification database provides access to the largest, most comprehensive list of qualified CSM providers. The reports provided by AABB allow biopharma organizations to find and verify sites faster at lower costs with the goal of expanding to commercialization with greater speed.
AABB’s CSM Qualification demonstrates that that a facility has a comprehensive quality management system that addresses:
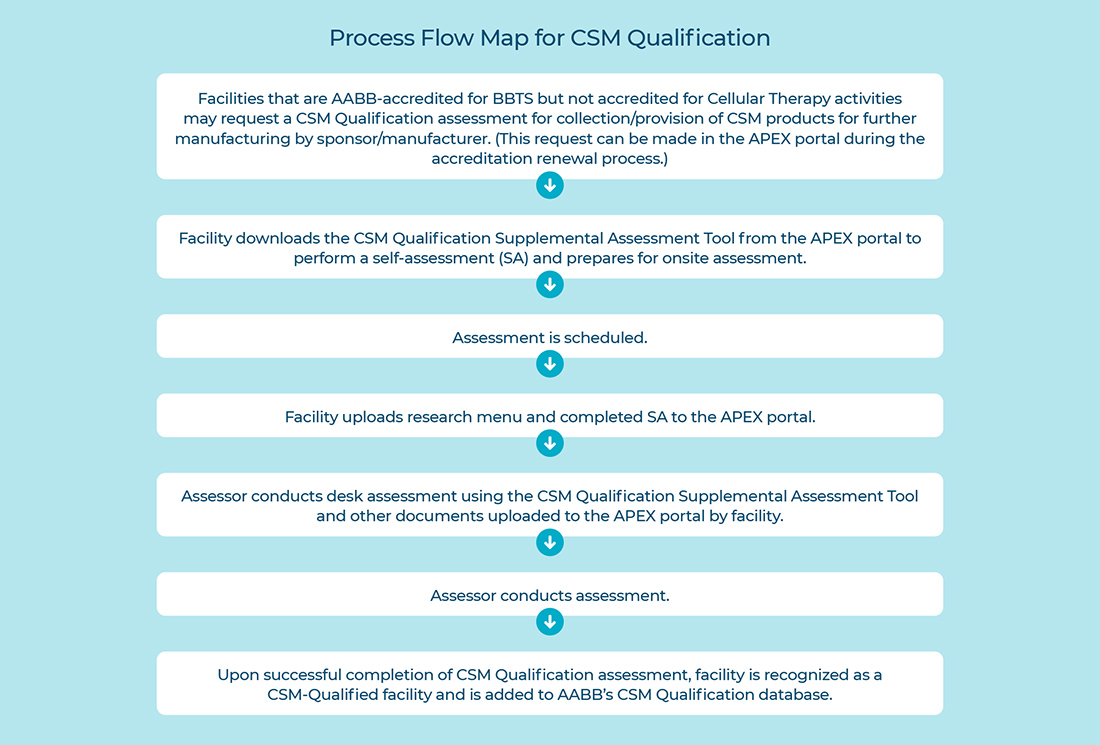
The CSM Qualification application can be found in the APEX portal, under the Accreditation Tab >Accrediting Bodies. There you will find an option for facility to select CSM Qualification. Once the selection is made, under the Documents Checklist > Accreditation tab, the facility is instructed to upload their research menu/list of products and the completed CSM Qualification supplemental assessment tool. Facilities should complete these documents before the assessment. Refer to the Process Flow Map for CSM Qualification program below for further clarification.
All are encouraged to apply. Facilities with an AABB-accredited blood collection service will be prioritized in the application process.
There are no additional fees associated with the CSM Qualification recognition for facilities that have a blood collection service accredited by AABB. Facilities that are not currently AABB-accredited may incur an additional fee for the assessment.
The assessor will provide a preliminary report at the completion of the assessment. After a review process by AABB staff, an official letter will be issued.
No, the CSM Qualification does not have any bearing on a facility’s accreditation status for the accredited activity.
Yes, a letter will be provided to your facility, as well as the CSM Qualification supplemental assessment tool used by the assessor, which will provide information on the standards to which your process did not conform.
Yes, in the same way that facilities must be reaccredited, the CSM Qualification must be chosen through the APEX portal every two years.
If this is what your facility prefers, simply email accreditation@aabb.org and your facility will be removed from the database.
Although this program has been rolled out so that CSM Qualification assessments are to be performed at the same time as a facility’s accreditation assessment, AABB will consider such requests on a case-by-case basis. Please email accreditation@aabb.org with such requests.
No, this program is for the collection of CSM products intended for further manufacturing, and which are collected and shipped for further processing to other facilities.
Facilities that are AABB-accredited for cellular therapies automatically qualify for this program and are listed in the database. They will be assessed automatically the next time their assessment is scheduled. There is no need to take any action.