
The CABP credentialing program evaluates a professional's knowledge across seven biotherapy domains, providing a standardized measure of knowledge, confidence in an individual's expertise, and validation of that professional's abilities.
In order to become a CABP, candidates must:





The CABP exam is open to all experienced biotherapies professionals, including, but not limited to:
For more information on the exam including eligibility and how to apply, please follow the link below.
In this article series, AABB interviews and highlights Certified Advanced Biotherapies Professionals. Read as they share what inspired them to pursue the CABP certification and how this new program will help address current challenges in the biotherapies field.
Alternatively, to view the full registry of CABPs follow the link below:
Developed by AABB in response to worldwide workforce shortages in the industry and the challenges associated with identifying and retaining qualified employees, the CABP certification benefits the entire biotherapies field by:
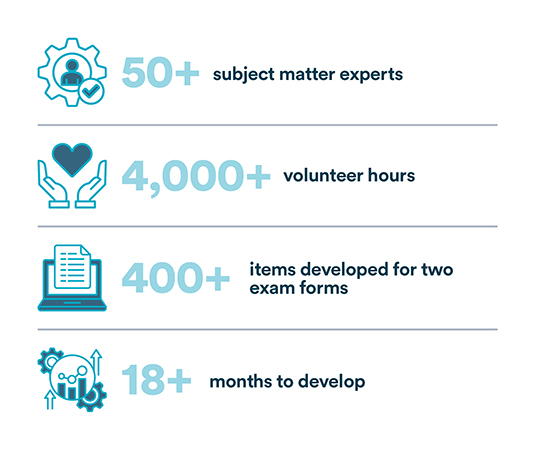
"The CABP exam was established through a rigorous and methodical process in accordance with the ISO/IEC 17024 standard for personnel certification. It is designed to validate a candidates’ knowledge, experience, and qualifications, and was developed with the input of more than 50 subject matter experts in the field who contributed more than 4,000 hours to the effort."
Jackie Thomas
VP Education, AABB
 First CABP application received.
First CABP application received.
 First cohort of CABPs certified.
First cohort of CABPs certified.
Meet six Certified Advanced Biotherapies Professionals (CABP)s working across different areas in the field as they share what motivated them to pursue the certification, how they see it impacting their work, their organization and the field as a whole.


