
The CABP is a pioneering credentialing exam within the biotherapies field, specifically designed to help identify high-caliber professionals working in the cell and gene therapy field.
Earning the CABP is a mark of distinction and contributes to the advancement of the field by demonstrating a high standard of competence across key biotherapies-related domains of knowledge. By taking the CABP exam and demonstrating the commitment to lifelong learning through recertification, CABPs represent a pool of qualified and proficient professionals throughout the world, ready to advance innovation, safety and quality in the field.
In order to become a CABP, candidates must:





Complete your CABP application and, upon approval, receive a special offer from AABB: Free CABP Exam Prep Bootcamps video series (a $275 value).
All CABP applications subject to AABB approval. AABB reserves the right to end this offer at any time.
This certification is intended for experienced professionals working in advanced biotherapies. Example roles include:
For more information on the exam including eligibility and how to apply, please follow the link below.
In this article series, AABB interviews and highlights Certified Advanced Biotherapies Professionals. Read as they share what inspired them to pursue the CABP certification and how this new program will help address current challenges in the biotherapies field.
Alternatively, to view the full registry of CABPs follow the link below:
Developed by AABB in response to worldwide workforce shortages in the industry and the challenges associated with identifying and retaining qualified employees, the CABP certification benefits the entire biotherapies field by:
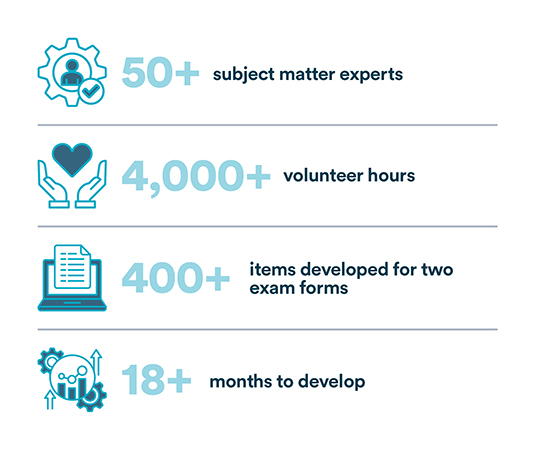
"The CABP exam was established through a rigorous and methodical process in accordance with the ISO/IEC 17024 standard for personnel certification. It is designed to validate a candidates’ knowledge, experience, and qualifications, and was developed with the input of more than 50 subject matter experts in the field who contributed more than 4,000 hours to the effort."
Jackie Thomas
VP Education, AABB
 First CABP application received.
First CABP application received.
 First cohort of CABPs certified.
First cohort of CABPs certified.
 AABB releases CABP Sample Exam Question sets and CABP Resource Library.
AABB releases CABP Sample Exam Question sets and CABP Resource Library.


