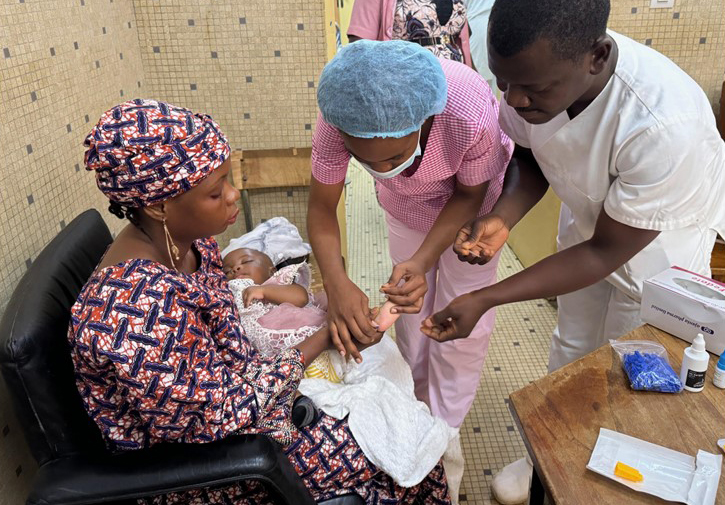
In this issue, AABB News spotlights three pioneering leaders in the blood and biotherapies community who are at the forefront of sickle cell disease research and advancing the treatment of the disease worldwide.

Joseph Lubega, MD, MPH, CPE, a specialist in childhood cancer and blood diseases at Texas Children’s Hospital in Houston, Texas, and associate professor at Baylor College of Medicine, is on a mission to change the landscape of SCD care for children in low- and middle-income countries (LMICs).
Lubega noted that Texas Children’s has been involved in global health for more than 25 years, particularly in Africa. Texas Children’s and Baylor led some of the first successful HIV treatment programs for children in Africa during the height of the HIV/AIDS epidemic, debunking the assumption that children with HIV in low-resource settings had little or no chance of survival. The successful initiative—which provided care for more than 396,000 mothers and children across the region—laid the groundwork for establishing a global program to treat children living with other complex diseases in underserved regions.
“We truly feel an obligation to support pediatrics globally because the majority of the world's children don't live in North America or Europe; they live in LMICs,” Lubega said. “This gives us a strong sense of responsibility for the health of children beyond Houston and the U.S.”
In 2017, Texas Children’s launched the Global HOPE (Hematology-Oncology Pediatric Excellence) program to improve the treatment of pediatric cancer and blood disorders in children in sub-Saharan Africa. Earlier this year, Global HOPE and Baylor, with support of Bristol-Myers Squibb Foundation, launched a groundbreaking initiative to address the high burden of SCD throughout the region, expanding sickle cell care from tertiary centers to communities. The program brings together African and American medical, public health and administration professionals with expertise in SCD, global health, public health, health education, implementation science, health cost analysis, project management, monitoring and evaluation, public relations, marketing and philanthropy.

“We’ve trained 36 specialists who have transformed outcomes for children. They all recognize that, while SCD is considered rare in the U.S., it's endemic in Africa,” he said. “These children endure constant pain crises, and by the age of five, 50% to 80% have died. The disease devastates families, communities and economies. There's also a heavy stigma, especially toward mothers, because it’s a genetic condition.”
Lubega emphasized the importance of building strong partnerships to scale up life-saving interventions across the continent, noting his institution's SCD initiative relies on collaboration with governments/ministries of health, foundations, pharmaceutical companies, the Africa CDC and local communities. “We engage families and people with SCD to guide our approach,” he said. “We must understand their perspective and what's important to them.”
A study published in Blood reported that Ugandan children with sickle cell anemia had 60% fewer severe or invasive infections, including malaria, after starting hydroxyurea treatment.3 In LMICs, people with SCD face barriers to accessing disease-modifying treatments like transfusions and hydroxyurea, which are essential for managing the disease and reducing complications.4 The Global HOPE’s initiative aims to integrate proven, high-impact interventions into primary child health services, including early screening, vaccinations, daily penicillin and hydroxyurea.
Lubega described these interventions as inexpensive, feasible and life-saving. None require advanced medical infrastructure, and they can be implemented even in rural clinics by nurses, physician assistants or family doctors, he pointed out.
“We’ve learned from our experience in the U.S. that the most impactful changes in SCD outcomes came from basic interventions—not expensive treatments,” Lubega said. “The 1972 National Sickle Cell Anemia Control Act mandated screening, increasing life expectancy in America. And the introduction of these interventions further improved survival. We want to replicate these proven steps in Africa.”
Lubega identified four key pillars to scale these interventions: stakeholder engagement, training for health care workers, accessible supply of medicines and test kits and a digital infrastructure with real-time data tracking, communication and program monitoring. Additionally, governments, communities, families affected by SCD and pharmaceutical companies must come together to address the problem and drive change, he added.
“Neglecting sickle cell care in Africa affects everyone. Because the largest population of affected individuals remains untreated, the disease lacks sufficient global investment,” he said. “Treatments like gene therapy—costing millions—remain out of reach and unscalable. If we can build a broader market, we can reduce costs and drive innovation that benefits patients globally.”

“In the next five years, I hope the question is no longer, “Can we do this?” but “How do we reach more kids?” Lubega said. “I want African institutions to be leading, with us in a supporting role.”
Texas Children’s was recently named one of the top five finalists for the MacArthur Foundation's 100&Change grant, a global competition awarding a single $100 million grant to help solve one of the world’s most critical social challenges. Lubega expressed his excitement about the opportunity, adding that the grant would help to secure long-term sustainability and strengthen fragile health systems beyond just sickle cell care. The biggest challenge, he said, is sustainability. Governments must invest in these services when external funding ends.
“This grant would allow us to scale quickly, train thousands of health workers and supply medicines across Africa. The momentum is already there—governments are eager, and communities are ready,” Lubega told AABB News. “SCD is a social justice issue. The disease predominantly affects Black people, as well as children in impoverished regions who have no voice. The MacArthur grant would be a powerful commitment to those children.”
Despite the current landscape of SCD in sub-Saharan Africa, including the alarmingly high mortality rate, Lubega remains hopeful about the future of SCD treatment.
"Ten years from now, I envision a world where every child with SCD has access to care, and life expectancy is no longer cut short by a manageable disease,” Lubega said. “I also hope global access makes it economically feasible to scale up novel treatments like gene therapy and reduce costs dramatically so that SCD becomes a curable disease as opposed to a lifelong disease.”

Thompson, who has dedicated her career to sickle cell research, spoke to AABB News about the challenges of treating SCD in LMICs, as well as implementing gene therapy. She noted there is not a one-size-fits-all approach to address the global health issue.
“There's still widespread concern that patients in many LMICs aren't receiving effective interventions like early diagnosis and access to hydroxyurea in a standardized way,” Thompson said. “There's also a fear that managing SCD could overwhelm health care systems, but we must balance these concerns with the devastating consequences of not treating the disease.”
Although the Lancet Hematology Commission endorses population-based public health priorities like newborn screening, it states the ultimate aim is to provide access to definitive or curative therapies (including potentially some form of gene therapy), for every patient with SCD, regardless of geographical location. This commission recognizes that widespread use of curative gene therapy will require early planning and strongly recommends prioritizing ongoing scientific and infrastructure investment to achieve effective, safe and accessible cures globally by 2040.5
Financial resources are a significant burden, but not the only hurdle, Thompson pointed out. Another important issue is follow-through. “The responsibility for implementation and accountability is often overlooked,” she said. “Even with detailed recommendations like those from the Lancet Hematology Commission, who ensures that these are acted upon? And is there an entity responsible for tracking implementation?”
Thompson emphasized the need for national plans in countries with a high burden of SCD, as these plans provide a framework for governments and key stakeholders to determine priorities and allocate resources. Creating these plans typically involves collaboration among public health officials, epidemiologists, academicians and government representatives, she explained.
“We’ve seen the value of a limited number of national plans in some sub-Saharan African countries, which could help inform insurance coverage and essential medicine lists. But without that national framework, it's hard to make significant progress in expanding access,” Thompson said. “Ultimately, however, implementation must happen within each individual country.”
As a pioneering researcher in SCD treatment and collaborator in groundbreaking clinical trials, Thompson travels the world to share insights on the transformational impact of gene therapy on individuals with SCD. In countries with resources, the outcomes from both commercial and investigational gene therapies have been promising, durable and improving lives, Thomas pointed out.
Although science is progressing rapidly, logistical infrastructure remains a significant barrier in many regions. A key missing piece is blood banking. Thompson noted that blood banking infrastructure is crucial for implementing gene therapy, particularly in sub-Saharan Africa where national blood systems may be lacking. Having the capacity to safely isolate and store blood components like platelets is essential for gene therapy and transplant.
“Many national plans for SCD don't include blood banking, though some countries have separate initiatives for standardizing blood services. But undoubtedly, blood banking will become the cornerstone for current gene therapies,” Thompson told AABB News. “Without reliable infrastructure—especially blood services—gene therapy becomes almost impossible to implement, particularly in regions without national blood systems or with limited voluntary blood donations.” Furthermore, widespread access, especially in places like sub-Saharan Africa, will be extremely difficult.
The longstanding silence around SCD treatment has shifted over the last decade. Thompson credited the increased attention to a wave of innovation—more drug options, transplants for people without sibling donors and young professionals who are building their careers around SCD. She expressed her enthusiasm about the prioritization of SCD, as well as growing engagement and support both within and beyond hematology. Having a global perspective on gene therapy topics is critical, Thompson added.
“Colleagues who previously focused on other areas are now realizing they can make a real impact here,” she said. “It's inspiring to see this collective effort, engaging European colleagues, and governmental bodies in high-burden countries and ministries of health, and witnessing a growing desire for conversation and progress on SCD, even in countries with many competing priorities. It can be easy to stay focused on the U.S., or Europe, but these conversations are happening worldwide.”
Thompson also highlighted the importance of collaboration between hematology and transfusion medicine specialists. “There's been a long-standing and deeply valuable partnership between both fields in the SCD space that is immensely valuable,” Thompson said. “People like Stella Chou, Dave Friedman, Sally Campbell-Lee and others are doing groundbreaking work with blood products and refining transfusion protocols to better manage SCD. Their efforts go beyond gene therapy.”
Although Thompson acknowledged the need for education and screening in LMICs, she stressed the importance of respecting and supporting, “the voices of our colleagues in those regions rather than imposing our own solutions, even while being pragmatic.”
“When I speak with my colleagues there, they express a strong desire to explore pathways to advance technologies on the continent. They’re interested in building expertise in blood banking and getting transplants on their countries’ essential medicines lists, so they can provide safer products,” Thompson said. “We want to make sure their voices drive our collective efforts around SCD.”

“There weren’t many practitioners in Charleston, S.C., caring for children with SCD then, so she became a cornerstone for the community,” Anderson said. “The way she connected with patients and their families and understood their social realities profoundly impacted me. I learned SCD treatment is about more than just the disease—it’s a partnership with families to make a difference in the management of the condition.”
Anderson’s commitment to caring for children with SCD took on a global focus when he discovered that Koutiala Hospital for Women and Children, a small rural facility in Mali, was not seeing any children with the disease, despite the high prevalence of SCD in the region.
“My former colleague, who worked at Koutiala Hospital, and I hypothesized that children were dying undiagnosed, as research shows 50–80% of children with SCD in sub-Saharan Africa die before age 5 from preventable causes,” he said. “So, we asked ourselves, what can we do?”
Research shows that early intervention through newborn screening can significantly reduce mortality and improve quality of life for affected children. In North America, newborn screening is integrated as part of routine universal public health programs; however, similar initiatives in Africa have been less effective because of limited coverage.4
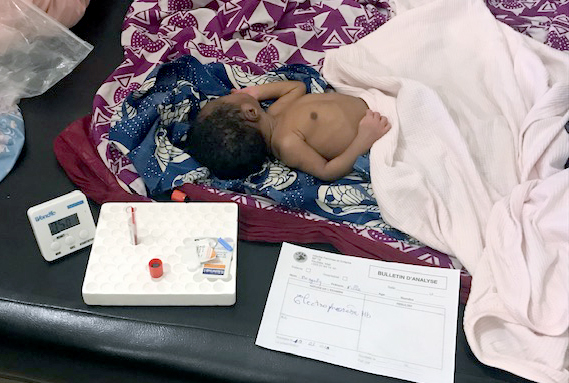
The ongoing project transitioned to rapid point-of-care (POC) tests to improve scalability in 2019 and currently screens approximately 3,000 babies per year. To date, more than 20,000 infants have been screened at Koutiala Hospital, and 750 infants diagnosed with SCD are linked to life-saving care in Mali and Togo.
“Our mission is to ensure every child in sub-Saharan Africa has access to a diagnosis and critical, life-saving care,” Anderson said. “We know that without diagnosis, outcomes are often dismal—but with low-cost interventions, we can drastically change that, even in low-resource settings.”
In 2023, Anderson co-published an article in Blood analyzing the ongoing POC newborn screening program in Koutiala, Mali. Between March 2019 and March 2023, 11,938 infants were born at Koutiala Women's and Children's Hospital, and 11,818 (99%) were offered screening for SCD using the HemoTypeSC test. SCD was diagnosed in 181 infants (1.54%), all of whom were referred to the hospital’s SCD treatment program for ongoing basic care. The study concluded that the low cost and rapidity of POC testing led to a high rate of newborn screening and effective clinical follow-up.6
Following the success of the POC testing at Koutiala Hospital, Anderson recognized the self-funding strategy would not be sustainable for expanding screening in Mali and launching new programs throughout the region. To that end, he established Sickle Forward in 2023 to expand screening partnerships and build sustainable programs in sub-Saharan Africa.
Since its inception, Sickle Forward has expanded to partnerships with local national government organizations and sickle cell centers, launching large-scale, grant-supported screening and treatment projects in Togo, Uganda and Tanzania, with upcoming projects in Cameroon, Gabon and Benin. None of this would have been possible without funding partners and local support, Anderson pointed out.
“From the beginning, we emphasized that we do not own any data collected—this work belongs to the local people and national programs,” Anderson said. “We want in-country leadership to guide the cultural sensitivity, decision-making, and ultimately, influence health policy.”
Anderson noted the Sickle Forward’s logo was inspired by Malian mud cloth art—depicting a woman carrying a child, symbolizing the burden mothers often carry when navigating SCD in their families. “We want our partnerships to represent the communities affected by the disease to improve access to timely diagnosis and basic, lifesaving care,” he said.
Sickle Forward aims to educate communities about SCD, address stigma and dispel myths (such as SCD being a curse). Awareness campaigns are launched before screening projects to establish rapport within the community. The organization also works with local partners to deliver culturally relevant, language-appropriate education to health care providers and families.
“We strive to empower families with knowledge. When parents understand what SCD is and how it's inherited, they’re better equipped to manage it and make future reproductive decisions,” Anderson said. “We hope this will ultimately reduce the number of new cases through informed choices.”

Although Sickle Forward’s core mission focuses on diagnosis and basic care, the organization strongly advocates for access to transformative therapies like gene therapy and bone marrow transplants. Its “Summits for Sickle Cell” hiking campaigns spotlight these advancements by including sickle cell warriors who have undergone these novel therapies.
“Sickle Forward will continue to advocate for both ends of the care spectrum—from transformative therapies to basic diagnostics and linkage to care, and events like ‘Summits for Sickle Cell’ allow us to do both,” Anderson said.
The organization’s inaugural summit, the 2024 Timmerman Transverse, brought together a diverse group of participants, including Jimi Olaghere, the first patient with SCD to summit Mount Kilimanjaro. The event raised more than $1.5 million, with proceeds supporting SCD research in the U.S. and advancing Sickle Forward’s work in Africa. The 2025 Summits for Sickle Cell in Colorado will feature four sickle cell warriors who have received transformative therapies, with a $500,000 fundraising goal.
“This year’s event is designed to celebrate our honorary warrior participants and these transformative therapies, as well as spotlight the vast number of people lacking access to basic care,” Anderson said. “It’s about elevating all levels of the SCD landscape, and that’s why I’m so excited about it. We hope to inspire supporters and demonstrate what’s possible after treatment.”
Looking ahead, Anderson said he is most excited about the biotech industry’s focus on developing new therapies for SCD and hopes to see increased funding and awareness for work beyond the U.S., particularly in sub-Saharan Africa, India and South America, where the disease is also prevalent.
“The vast majority of people with SCD worldwide won’t have access to gene therapy anytime soon,” Anderson said. "Our work ensures that children live long enough to one day benefit from advanced treatments.”
He also shared his desire to see more individuals with lived experience driving the conversation around SCD treatment. “When there’s hope, people rise up and become the loudest voices for progress,” Anderson told AABB News. “That’s what’s been missing in SCD—because of stigma, lack of awareness and inequity. But we believe that’s changing, and that those living with SCD will lead the charge for a better future.”
BACK TO ISSUE
June 2025
Transfusion is AABB’s scholarly, peer-reviewed monthly journal, publishing the latest on technological advances, clinical research and controversial issues related to transfusion medicine, blood banking, biotherapies and tissue transplantation. Access of Transfusion is free to all AABB members.
Learn More About Transfusion Journal
Keep abreast of what's happening in the field of biotherapies with CellSource - AABB's monthly update on the latest biotherapies news.
To submit news about the blood and biotherapies field to AABB, please email news@aabb.org.
President
Meghan Delaney, DO, MPH
Chief Executive Officer
Debra Ben Avram, FASAE, CAE
Chief Communications and Engagement Officer
Julia Zimmerman
Director of Marketing and Communications
Jay Lewis, MPH
Managing Editor
Kendra Y. Mims, MFA
Senior Communications Manager
Drew Case
AABB News
(ISSN 1523939X) is published monthly, except for the combined November/December issue for the members of AABB; 4550 Montgomery Avenue; Suite 700 North Tower; Bethesda, MD 20814.
AABB is an international, not-for-profit association representing individuals and institutions involved in transfusion medicine, cellular therapies and patient blood management. The association is committed to improving health by developing and delivering standards, accreditation and educational programs that focus on optimizing patient and donor care and safety.
+1.301.907.6977
Email: news@aabb.org
Website: www.aabb.org
Copyright 2025 by AABB.
Views and opinions expressed in AABB News are not necessarily endorsed by AABB unless expressly stated.
Notice to Copiers: Reproduction in whole or part is strictly prohibited unless written permission has been granted by the publisher. AABB members need not obtain prior permission if proper credit is given.
