AABB has over 60 years of experience in standards setting activities, and an accreditation program that encompasses multiple fields within healthcare. For over 20 years, AABB has been applying its core principles of quality and donor and patient safety to the field of cellular therapy through its prestigious Standards and Accreditation programs.
The AABB Accreditation Program is the only program of its kind in the United States that is itself 'accredited'. Accredited by International Society for Quality in Healthcare or ISQua, AABB has been recognized at an international level for 'quality.' ISQua is the leading global health care external evaluation program. AABB's standards are recognized as state law in California.
Using a quality systems approach, AABB Standards for Cellular Therapy Services covers all aspects of product collection, manufacture and patient care. Developed by a committee of experts, from a variety of organizations, including representatives from the United States Food and Drug Administration (FDA), the Standards for Cellular Therapy Services are designed to be broadly applicable to a range of activities, clinical programs, and cell types-- including but not limited to hematopoietic stem cell transplant services.
AABB, as a pioneering organization in the application of quality management systems and technical requirements for cellular therapy activities, accredits cellular therapy programs and facilities around the world. AABB accreditation has helped facilities lead the way to a reputation of outstanding quality.
AABB's Standards aims to promote patient, donor, and product safety compatible with the universally accepted International Organization for Standardization, or ISO, standards.
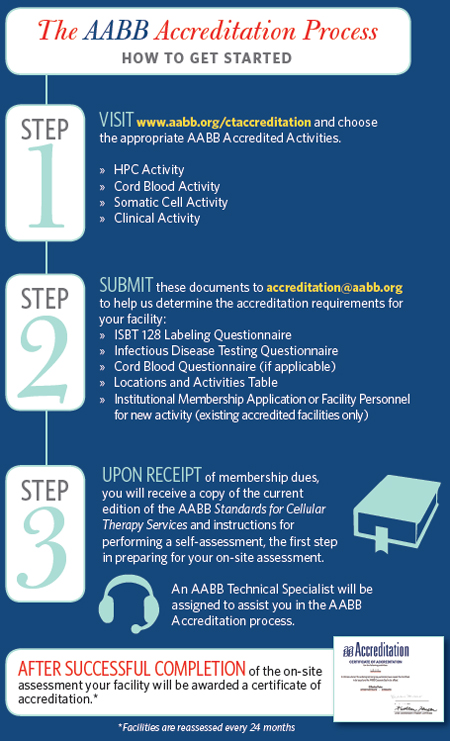
The Accreditation Program is designed to grant accreditation for specific activities, including a variety of cellular therapy activities, reflected on the accreditation certificate. The time involved in gaining accreditation will vary depending on each applicant's capacity to complete the steps in the process.
AABB Accepting Applications for 2026 Future Leader Scholarship Awards Program
March 03, 2026
FDA Seeks Comment on BPD, HCT/P Deviation Reporting
February 25, 2026
FDA Issues Draft Guidance Establishing Framework for Individualized Gene Therapies
February 25, 2026