

“My parents saw how much this crisis impacted me and worsened my quality of life,” he said. “They encouraged me to explore other options, so we tried a bone marrow transplant. Unfortunately, none of my sisters were a match.”
In 2019, Olaghere learned about gene therapy as a potential cure for SCD and set up a Google alert to keep abreast of new research and developments. Months later, he received an NPR article in his inbox about Victoria Gray, the first person to undergo CRISPR-based gene therapy for SCD. Gray’s story revived his hope and spurred him to reach out to the principal investigator of the clinical trial.
“That moment felt like divine timing,” he said. “My wife was eight months pregnant with our first child when we read the article. This opportunity serendipitously landed on my lap. My mindset shifted. I was no longer jaded or defeated but excited about life and hopeful about the future and fatherhood.”
Olaghere joined a CRISPR gene-editing clinical trial, called Casgevy, at Sarah Cannon Research Institute in Nashville, Tenn. As an entrepreneur, Olaghere noted he and his wife Amanda hired help for his business and established a support system to prepare for their three-month stay in Nashville during the pandemic. The time frame added to the feeling of being isolated, but in some ways, also more focused, Olaghere added.
Olaghere entered the trial with an open mind. His optimism outweighed any concerns about potential side effects or complications. “I was quite desperate. I just wanted the opportunity to live a normal life as a father, even if temporarily,” he said. “The other three participants were doing well, so I was not afraid. I believed the trial would give me an opportunity to experience a better quality of life.”
He began the months-long gene therapy process in January 2020. For Olaghere, cell collection and chemotherapy conditioning were the most grueling parts of the process; the side effects of chemotherapy hit him hard two weeks after the infusion. To manage blood loss during the process, he received a whole blood transfusion after each cell collection during the first phase, totaling nearly 30 units of blood. Olaghere expressed his appreciation for blood donors, adding that blood donations sustained him during critical parts of his treatment.
“I needed more blood when my counts dropped dangerously low during chemotherapy. These transfusions were essential,” he added.
Blood donations are imperative for supporting this patient population throughout the gene therapy process. “People don’t always understand the direct life-saving impact of blood donation. I’ve been in situations where doctors said I might not make it if I didn’t receive blood in 24 hours. Someone’s donation saved my life,” Olaghere said.
Olaghere completed gene therapy in September 2020. Two weeks after he was infused with his gene-edited cells, he noticed a significant difference in his body. The lingering pain he lived with for decades was gone.
“It felt like the disease was letting me go,” he said.
In 1985, Olaghere's parents, both carriers of the sickle cell trait, traveled to the United States from Nigeria for prenatal screening after a doctor explained there was a chance their unborn child could inherit the disease. The amniocentesis test confirmed that Olaghere would be born with SCD.
“My diagnosis was quite fortunate. I’m of Nigerian heritage, and children born in Nigeria with severe SCD often don’t live past the age of five if it's not diagnosed or managed early,” he explained. “Prenatal screening wasn’t accessible in Nigeria back then, so there was no way my parents could have known I’d have SCD until they came to Washington, D.C. Because of the positive result, they decided to give birth to me in the U.S. I honestly believe that decision saved my life.”
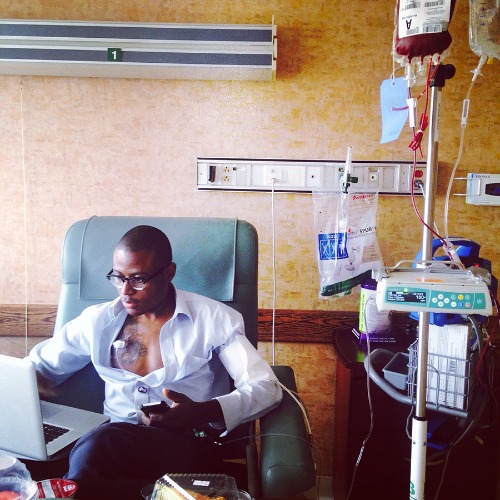
Olaghere has undergone numerous treatments throughout his life, including more than 200 blood transfusions, and endured various other health issues, including acute chest syndrome, pulmonary embolism, avascular necrosis, priapism, blindness, gallbladder removal and severe iron overload. He says he never experienced a day without pain until gene therapy. Since treatment, Olaghere’s quality of life has drastically improved. He has not faced a crisis or any SCD-related hospitalizations. The incessant chronic pain he suffered hasn't returned.
“My life before and after gene therapy is like night and day,” Olaghere told AABB News. “I went from staying in bed all day, wracked with pain and popping pills for relief, to being strong enough to climb summits. That shows how transformative gene therapy has been for me.”
In 2024, Olaghere, now 39, made history as the world’s first patient with SCD to summit Mount Kilimanjaro, Africa’s tallest mountain and the world’s highest free-standing mountain. He was invited to join 20 biotech leaders for the Timmerman Traverse 2024 in support of Sickle Forward. The team raised more than $1.25 million to advance SCD screening and treatment across sub-Saharan Africa.
This event was a major feat as patients with SCD are advised to avoid elevations of more than 10,000 feet. To prepare for the climb, Olaghere trained at higher altitudes and incorporated more “leg days” at the gym to improve endurance. “I had already been physically training to stay healthy after gene therapy. After 34 years of not having control of my body, I became obsessed with my physical health, so I was ready,” he said.
He added that focusing on the journey instead of the outcome helped him mentally prepare for the challenge.
“I remember thinking, it’s -16°F, and midnight, and I’m on my way to the tallest freestanding peak in the world,” he recalled. “I was so exhausted and just happy that we reached the summit. I was in shock that I had reached a place in my life where I could do that. It was surreal. That moment of reflection was overwhelming. There were so many emotions.”
Olaghere looks forward to participating in Sickle Forward’s Summits for Sickle Cell benefit this September in Colorado, where he will summit three mountain peaks with fellow sickle cell warriors and gene therapy recipients to raise funds to expand access to treatment in low-and middle-income countries. Equitable access to safe blood and fetal hemoglobin-inducing drugs is needed for those who don’t need or want gene therapy, he added.
“People often forget the epicenter of SCD is in sub-Saharan Africa, as well as in regions like India and the Middle East. Many patients on the outskirts of these major cities continue to suffer due to a lack of basic health care resources and care,” he stated. “This is why people with SCD are still dying in large numbers in low-resource settings. The health care system is severely broken. We need to do the work to improve the quality of life for these populations and ensure they stay alive until these gene therapies become more accessible.”
Many patients on the outskirts of these major cities continue to suffer due to a lack of basic health care resources and care.
Although Olaghere advocates for equitable access to gene therapy, he acknowledged that gene therapy is not the solution for every sickle cell warrior.
“Access is important, but my advocacy efforts are generally focused on ensuring a holistic approach to treating SCD globally and making sure patients and their families have the options they need to manage and ultimately overcome this disease,” Olaghere said.
His message for individuals with severe sickle cell disease without access: Do whatever you can to make it to the next day.
“I tell them it's a step-by-step battle. These gene therapies will become more widely available one day. Technology will proliferate and expand access to treatment options,” he said. “If gene therapy is something you want in the future, keep pushing to survive another day, and take it one day at a time.”
Olaghere also shared his growing passion for post-transplant support, highlighting the importance of providing warriors with significant care and resources after undergoing curative therapy. There’s a tendency to think the journey ends once you're “cured,” but there's still a lot of work to be done, he pointed out.
“We’re actively working with manufacturers and clinical trial sponsors to make sure they understand that support must continue well beyond the treatment,” Olaghere said.
The proud father of three discussed his personal commitment to keep showing up every day and doing his part to make gene therapies accessible to the patient populations that desperately need them. He is grateful for the opportunity to receive gene therapy and wants others to have access to the same transformative treatment.
“Because of gene therapy, I now have three children and a career path that I've always wanted to explore,” Olaghere told AABB News. “I'll continue to advocate and help in any way I can, while also remembering to take some time for myself along the way.”
BACK TO ISSUE
June 2025
Transfusion is AABB’s scholarly, peer-reviewed monthly journal, publishing the latest on technological advances, clinical research and controversial issues related to transfusion medicine, blood banking, biotherapies and tissue transplantation. Access of Transfusion is free to all AABB members.
Learn More About Transfusion Journal
Keep abreast of what's happening in the field of biotherapies with CellSource - AABB's monthly update on the latest biotherapies news.
To submit news about the blood and biotherapies field to AABB, please email news@aabb.org.
President
Meghan Delaney, DO, MPH
Chief Executive Officer
Debra Ben Avram, FASAE, CAE
Chief Communications and Engagement Officer
Julia Zimmerman
Director of Marketing and Communications
Jay Lewis, MPH
Managing Editor
Kendra Y. Mims, MFA
Senior Communications Manager
Drew Case
AABB News
(ISSN 1523939X) is published monthly, except for the combined November/December issue for the members of AABB; 4550 Montgomery Avenue; Suite 700 North Tower; Bethesda, MD 20814.
AABB is an international, not-for-profit association representing individuals and institutions involved in transfusion medicine, cellular therapies and patient blood management. The association is committed to improving health by developing and delivering standards, accreditation and educational programs that focus on optimizing patient and donor care and safety.
+1.301.907.6977
Email: news@aabb.org
Website: www.aabb.org
Copyright 2025 by AABB.
Views and opinions expressed in AABB News are not necessarily endorsed by AABB unless expressly stated.
Notice to Copiers: Reproduction in whole or part is strictly prohibited unless written permission has been granted by the publisher. AABB members need not obtain prior permission if proper credit is given.
