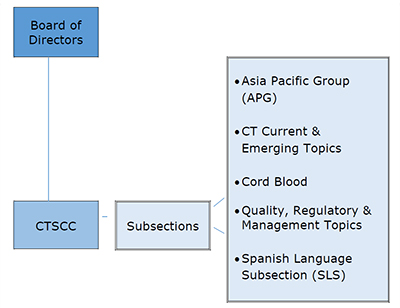
 The Cellular Therapies Section was established in 2009 to provide guidance to the association and its membership
on critical issues relating to cellular therapies, including umbilical cord blood, regenerative medicine and somatic cells. The Cellular Therapies Section is guided by a Coordinating Committee, or CTSCC, comprised of elected members with cellular
therapy expertise, that ensure that the Section meets the charges described below. The Chair of the CTSCC serves on the Board of Directors. Under the leadership of members of the CTSCC, subsections have been established. The subsections are comprised
of AABB members who have indicated their interest in subsection topics.
The Cellular Therapies Section was established in 2009 to provide guidance to the association and its membership
on critical issues relating to cellular therapies, including umbilical cord blood, regenerative medicine and somatic cells. The Cellular Therapies Section is guided by a Coordinating Committee, or CTSCC, comprised of elected members with cellular
therapy expertise, that ensure that the Section meets the charges described below. The Chair of the CTSCC serves on the Board of Directors. Under the leadership of members of the CTSCC, subsections have been established. The subsections are comprised
of AABB members who have indicated their interest in subsection topics.
This page includes information on the section charges. Please follow the links to learn more about the guidelines of the Cellular Therapies Section, the subsections
and how to join them, and the current members of the CTSCC.
Cellular Therapies Section Guidelines
Cellular Therapies Section Subsections
Cellular Therapies Section Coordinating Committee Roster
Frequently Asked Questions (FAQs) for Cellular Therapies Members
Center for Cellular Therapies
FDA Seeks Comment on BPD, HCT/P Deviation Reporting
February 25, 2026
FDA Issues Draft Guidance Establishing Framework for Individualized Gene Therapies
February 25, 2026
Japanese Regulatory Panel Recommends Advancing World’s First iPSC-Based Therapies
February 24, 2026